Thermodesulfobacteriota
| Thermodesulfobacteriota | |
|---|---|

| |
| Nitratidesulfovibrio vulgaris | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Pseudomonadati |
| Phylum: | Thermodesulfobacteriota Garrity & Holt 2021[1] |
| Classes[3] | |
| |
| Synonyms[3] | |
| |
The Thermodesulfobacteriota, or Desulfobacterota,[4] are a phylum of anaerobic Gram-negative bacteria. Many representatives are sulfate-reducing bacteria,[5] others can grow by disproportionation of various sulphur species,[6] reduction or iron,[7] or even use external surfaces as electron acceptors (exoelectrogens).[8] They have highly variable morphology: vibrio, rods, cocci,[4] as well as filamentous cable bacteria.[9] Individual members of Desulfobacterota are also studied for their bacterial nanowires[10] or syntrophic relationships.[11]

Taxonomy
[edit]The bacterial phylum Desulfobacterota has been created by merging: 1) the well-established class Thermodesulfobacteria, 2) the proposed phylum Dadabacteria, and 3) various taxa separated from the abandoned non-monophyletic class "Deltaproteobacteria" alongside three other phyla: Myxococcota, Bdellovibrionota, and SAR324.[4]
Environment
[edit]In contrast to their close relatives, the aerobic phyla Myxococcota and Bdellovibrionota, Desulfobacterota are predominantly anaerobic.[4] They likely retained their anaerobic lifestyle since before the Great Oxidation Event.[13]
Three closely related classes within Desulfobacterota: Thermodesulfobacteria, Dissulfuribacteria, and Desulfofervidia,[11] as well as the more distant Deferrisomatia, are exclusively thermophilic, while most members of other classes are mesophiles[4] or even psychrophiles.[14][15]
Metabolism
[edit]Sulfate-reducing bacteria (SRB) utilize sulfate as a terminal electron acceptor in a respiratory-type metabolism, coupled to the oxidation of organic compounds or hydrogen. By reducing sulfate, many Desulfobacterota species substantially contribute to the sulfur cycle.[4]

Microbial sulfur disproportionation (MSD) is a poorly known type of energy metabolism analogous to organic fermentation, where a single inorganic sulfur species of intermediate oxidation state is simultaneously oxidized and reduced, resulting in production of sulfide and sulfate. In Desulfobacterota, MSD is often present in species that also perform sulfate reduction.[6]
Fe(III) minerals can be microbially reduced by Fe-reducing bacteria (FeRB) using a wide range of organic compounds or H2 as electron donors. FeRB are widespread across Bacteria. Among Desulfobacterota, they are represented e.g. by the genus Geobacter (Desulfuromonadia).[16]
Certain species of the families Geobacteraceae and Desulfuromonadaceae (Desulfuromonadia) are able to use external surfaces as electron acceptors to complete respiration.[8][17][18] Species of the genus Geobacter use bacterial nanowires to transfer electrons to extracellular electron acceptors such as Fe(III) oxides.[10]

Certain species of the class Syntrophia use simple organic molecules as electron donors and grow only in the presence of H2/formate-utilizing partners (methanogens or Desulfovibrio) in syntrophic associations.[19]
The family Desulfobulbaceae contains two genera of cable bacteria: Ca. Electronema and Ca. Electrothrix. These filamentous bacteria conduct electricity across distances over 1 cm, which allows them to connect distant sources of electron donors and electron acceptors.[9]
-
Cable bacteria in between two layers of sediment
-
Diagram demonstrating cable bacteria metabolism in surface sediment
-
Model representation of a cable bacteria cell
Notable species
[edit]- Desulfobulbus propionicus (Desulfobulbia), described in 1982 from mud samples in Germany, can serve as a biocatalyst in microbial electrosynthesis, i.e. the usage of electrons by microorganism to reduce carbon dioxide to organic molecules.[20]
- Lawsonia intracellularis (Desulfovibrionia), described in 1995 from the intestines of pigs with proliferative enteropathy disease, is a highly pathogenic intracellular parasite.[21][22]
- Oleidesulfovibrio alaskensis (Desulfovibrionia), described in 2004 from an oil well in Alaska, corrodes metals and reduces toxic radionuclides and metals such as uranium and chromium to less soluble and less toxic forms.[23][24]
- Syntrophorhabdus aromaticivorans (Syntrophorhabdia), described in 2008 from industrial wastewater, is the first cultured anaerobe capable of degrading phenol to acetate in obligate syntrophic associations with a hydrogenotrophic methanogen.[25]
- Dissulfuribacter thermophilus (Dissulfuribacteria), described in 2013 from a deep-sea hydrothermal vent, does not reduce sulfate and instead disproportionates elemental sulfur and other intermediate sulfur species.[26]
- Ca. Desulfofervidus auxilii (Ca. Desulfofervidia), described in 2016 from Guaymas Basin sediments, is involved in the anaerobic oxidation of methane (AOM) together with archaeal syntrophic partners.[11]
Microscopy
[edit]-
Ca. Electronema sp.
Phylogeny
[edit]The phylogeny is based on phylogenomic analysis:
| 120 marker proteins based GTDB 09-RS220[28][29][30] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Waite et al. 2020[3]
|
|
See also
[edit]- Sulfur cycle
- Exoelectrogen
- Cable bacteria
- Syntrophy
- Thermophile
- Bacterial nanowires
- Sulfate reducing bacteria
Reference
[edit]- ^ Oren A, Garrity GM (2021). "Valid publication of the names of forty-two phyla of prokaryotes". Int J Syst Evol Microbiol. 71 (10): 5056. doi:10.1099/ijsem.0.005056. PMID 34694987.
- ^ Gavriilidou, et al. (2023). "Candidatus Nemesobacterales is a sponge-specific clade of the candidate phylum Desulfobacterota adapted to a symbiotic lifestyle". The ISME Journal. 17 (11): 1808–1818. doi:10.1038/s41396-023-01484-z. PMC 10579324. PMID 37587369.
- ^ a b c Waite DW, Chuvochina M, Pelikan C, Parks DH, Yilmaz P, Wagner M, Loy A, Naganuma T, Nakai R, Whitman WB, Hahn MW, Kuever J, Hugenholtz P. (2020). "Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities". Int J Syst Evol Microbiol. 70 (11): 5972–6016. doi:10.1099/ijsem.0.004213. PMID 33151140.
- ^ a b c d e f Waite, David W; Chuvochina, Maria; Pelikan, Claus; Parks, Donovan H; Yilmaz, Pelin; Wagner, Michael; Loy, Alexander; Naganuma, Takeshi; Nakai, Ryosuke; Whitman, William B; Hahn, Martin W; Kuever, Jan; Hugenholtz, Philip (2020-11-01). "Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities". International Journal of Systematic and Evolutionary Microbiology. 70 (11): 5972–6016. doi:10.1099/ijsem.0.004213. ISSN 1466-5026. PMID 33151140.
- ^ Müller, Albert Leopold; Kjeldsen, Kasper Urup; Rattei, Thomas; Pester, Michael; Loy, Alexander (2015-05-01). "Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi)sulfite reductases". The ISME Journal. 9 (5): 1152–1165. doi:10.1038/ismej.2014.208. ISSN 1751-7362. PMC 4351914. PMID 25343514.
- ^ a b Slobodkin, A. I.; Slobodkina, G. B. (2019). "Diversity of Sulfur-Disproportionating Microorganisms". Microbiology. 88 (5): 509–522. doi:10.1134/S0026261719050138. ISSN 0026-2617.
- ^ Slobodkina, G. B.; Reysenbach, A.-L.; Panteleeva, A. N.; Kostrikina, N. A.; Wagner, I. D.; Bonch-Osmolovskaya, E. A.; Slobodkin, A. I. (2012-10-01). "Deferrisoma camini gen. nov., sp. nov., a moderately thermophilic, dissimilatory iron(III)-reducing bacterium from a deep-sea hydrothermal vent that forms a distinct phylogenetic branch in the Deltaproteobacteria". International Journal of Systematic and Evolutionary Microbiology. 62 (Pt_10): 2463–2468. doi:10.1099/ijs.0.038372-0. ISSN 1466-5026. PMID 22140176.
- ^ a b Bond, Daniel R.; Holmes, Dawn E.; Tender, Leonard M.; Lovley, Derek R. (2002-01-18). "Electrode-Reducing Microorganisms That Harvest Energy from Marine Sediments". Science. 295 (5554): 483–485. doi:10.1126/science.1066771. ISSN 0036-8075. PMID 11799240.
- ^ a b Pfeffer, Christian; Larsen, Steffen; Song, Jie; Dong, Mingdong; Besenbacher, Flemming; Meyer, Rikke Louise; Kjeldsen, Kasper Urup; Schreiber, Lars; Gorby, Yuri A.; El-Naggar, Mohamed Y.; Leung, Kar Man; Schramm, Andreas; Risgaard-Petersen, Nils; Nielsen, Lars Peter (2012). "Filamentous bacteria transport electrons over centimetre distances". Nature. 491 (7423): 218–221. doi:10.1038/nature11586. ISSN 0028-0836. PMID 23103872.
- ^ a b Reguera, Gemma; McCarthy, Kevin D.; Mehta, Teena; Nicoll, Julie S.; Tuominen, Mark T.; Lovley, Derek R. (2005). "Extracellular electron transfer via microbial nanowires". Nature. 435 (7045): 1098–1101. doi:10.1038/nature03661. ISSN 0028-0836. PMID 15973408.
- ^ a b c Krukenberg, Viola; Harding, Katie; Richter, Michael; Glöckner, Frank Oliver; Gruber-Vodicka, Harald R.; Adam, Birgit; Berg, Jasmine S.; Knittel, Katrin; Tegetmeyer, Halina E.; Boetius, Antje; Wegener, Gunter (2016). "Candidatus Desulfofervidus auxilii, a hydrogenotrophic sulfate-reducing bacterium involved in the thermophilic anaerobic oxidation of methane". Environmental Microbiology. 18 (9): 3073–3091. doi:10.1111/1462-2920.13283. ISSN 1462-2912. PMID 26971539.
- ^ Martinez-Gutierrez, Carolina A; Aylward, Frank O (2021-12-09). Battistuzzi, Fabia Ursula (ed.). "Phylogenetic Signal, Congruence, and Uncertainty across Bacteria and Archaea". Molecular Biology and Evolution. 38 (12): 5514–5527. doi:10.1093/molbev/msab254. ISSN 1537-1719. PMC 8662615. PMID 34436605.
- ^ Davín, Adrián A.; Woodcroft, Ben J.; Soo, Rochelle M.; Morel, Benoit; Murali, Ranjani; Schrempf, Dominik; Clark, James; Boussau, Bastien; Moody, Edmund R. R. (2023-08-11), An evolutionary timescale for Bacteria calibrated using the Great Oxidation Event, doi:10.1101/2023.08.08.552427, retrieved 2025-03-27
- ^ Knoblauch, Christian; Sahm, Kerstin; Jørgensen, Bo B. (1999-10-01). "Psychrophilic sulfate-reducing bacteria isolated from permanently cold Arctic marine sediments: description of Desulfofrigus oceanense gen. nov., sp. nov., Desulfofrigus fragile sp. nov., Desulfofaba gelida gen. nov., sp. nov., Desulfotalea psychrophila gen. nov., sp. nov. and Desulfotalea arctica sp. nov". International Journal of Systematic and Evolutionary Microbiology. 49 (4): 1631–1643. doi:10.1099/00207713-49-4-1631. ISSN 1466-5026. PMID 10555345.
- ^ Isaksen, M. F.; Teske, Andreas (1996-09-13). "Desulforhopalus vacuolatus gen. nov., sp. nov., a new moderately psychrophilic sulfate-reducing bacterium with gas vacuoles isolated from a temperate estuary". Archives of Microbiology. 166 (3): 160–168. doi:10.1007/s002030050371. ISSN 0302-8933.
- ^ Luef, Birgit; Fakra, Sirine C; Csencsits, Roseann; Wrighton, Kelly C; Williams, Kenneth H; Wilkins, Michael J; Downing, Kenneth H; Long, Philip E; Comolli, Luis R; Banfield, Jillian F (2013-02-01). "Iron-reducing bacteria accumulate ferric oxyhydroxide nanoparticle aggregates that may support planktonic growth". The ISME Journal. 7 (2): 338–350. doi:10.1038/ismej.2012.103. ISSN 1751-7362. PMC 3554402. PMID 23038172.
- ^ Holmes, Dawn E.; Nicoll, Julie S.; Bond, Daniel R.; Lovley, Derek R. (2004). "Potential Role of a Novel Psychrotolerant Member of the Family Geobacteraceae , Geopsychrobacter electrodiphilus gen. nov., sp. nov., in Electricity Production by a Marine Sediment Fuel Cell". Applied and Environmental Microbiology. 70 (10): 6023–6030. doi:10.1128/AEM.70.10.6023-6030.2004. ISSN 0099-2240. PMC 522133. PMID 15466546.
- ^ Reguera, Gemma; McCarthy, Kevin D.; Mehta, Teena; Nicoll, Julie S.; Tuominen, Mark T.; Lovley, Derek R. (2005). "Extracellular electron transfer via microbial nanowires". Nature. 435 (7045): 1098–1101. doi:10.1038/nature03661. ISSN 0028-0836. PMID 15973408.
- ^ Kuever, Jan (2014), Rosenberg, Eugene; DeLong, Edward F.; Lory, Stephen; Stackebrandt, Erko (eds.), "The Family Syntrophaceae", The Prokaryotes, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 281–288, doi:10.1007/978-3-642-39044-9_269, ISBN 978-3-642-39043-2, retrieved 2025-03-27
- ^ Gong, Yanming; Ebrahim, Ali; Feist, Adam M.; Embree, Mallory; Zhang, Tian; Lovley, Derek; Zengler, Karsten (2013-01-02). "Sulfide-Driven Microbial Electrosynthesis". Environmental Science & Technology. 47 (1): 568–573. doi:10.1021/es303837j. ISSN 0013-936X. PMID 23252645.
- ^ McORIST, S.; Gebhart, C. J.; Boid, R.; Barns, S. M. (1995-10-01). "Characterization of Lawsonia intracellularis gen. nov., sp. nov., the Obligately Intracellular Bacterium of Porcine Proliferative Enteropathy". International Journal of Systematic Bacteriology. 45 (4): 820–825. doi:10.1099/00207713-45-4-820. ISSN 0020-7713. PMID 7547305.
- ^ Gebhart, C. J.; Barns, S. M.; Mcorist, S.; Lin, G.-F.; Lawson, G. H. K. (1993-07-01). "Ileal Symbiont Intracellularis, an Obligate Intracellular Bacterium of Porcine Intestines Showing a Relationship to Desulfovibrio Species". International Journal of Systematic Bacteriology. 43 (3): 533–538. doi:10.1099/00207713-43-3-533. ISSN 0020-7713. PMID 8347512.
- ^ Feio, M. J. (2004-09-01). "Desulfovibrio alaskensis sp. nov., a sulphate-reducing bacterium from a soured oil reservoir". International Journal of Systematic and Evolutionary Microbiology. 54 (5): 1747–1752. doi:10.1099/ijs.0.63118-0. hdl:21.11116/0000-0001-D111-F. ISSN 1466-5026. PMID 15388739.
- ^ Hauser, Loren J.; Land, Miriam L.; Brown, Steven D.; Larimer, Frank; Keller, Kimberly L.; Rapp-Giles, Barbara J.; Price, Morgan N.; Lin, Monica; Bruce, David C.; Detter, John C.; Tapia, Roxanne; Han, Cliff S.; Goodwin, Lynne A.; Cheng, Jan-Fang; Pitluck, Samuel (2011-08-15). "Complete Genome Sequence and Updated Annotation of Desulfovibrio alaskensis G20". Journal of Bacteriology. 193 (16): 4268–4269. doi:10.1128/JB.05400-11. ISSN 0021-9193. PMC 3147700. PMID 21685289.
- ^ Qiu, Yan-Ling; Hanada, Satoshi; Ohashi, Akiyoshi; Harada, Hideki; Kamagata, Yoichi; Sekiguchi, Yuji (2008). "Syntrophorhabdus aromaticivorans gen. nov., sp. nov., the First Cultured Anaerobe Capable of Degrading Phenol to Acetate in Obligate Syntrophic Associations with a Hydrogenotrophic Methanogen". Applied and Environmental Microbiology. 74 (7): 2051–2058. doi:10.1128/AEM.02378-07. ISSN 0099-2240. PMC 2292594. PMID 18281436.
- ^ Slobodkin, A. I.; Reysenbach, A.-L.; Slobodkina, G. B.; Kolganova, T. V.; Kostrikina, N. A.; Bonch-Osmolovskaya, E. A. (2013-06-01). "Dissulfuribacter thermophilus gen. nov., sp. nov., a thermophilic, autotrophic, sulfur-disproportionating, deeply branching deltaproteobacterium from a deep-sea hydrothermal vent". International Journal of Systematic and Evolutionary Microbiology. 63 (Pt_6): 1967–1971. doi:10.1099/ijs.0.046938-0. ISSN 1466-5026. PMID 23024145.
- ^ Fu, Tianda; Liu, Xiaomeng; Gao, Hongyan; Ward, Joy E.; Liu, Xiaorong; Yin, Bing; Wang, Zhongrui; Zhuo, Ye; Walker, David J. F.; Joshua Yang, J.; Chen, Jianhan; Lovley, Derek R.; Yao, Jun (2020-04-20). "Bioinspired bio-voltage memristors". Nature Communications. 11 (1): 1861. doi:10.1038/s41467-020-15759-y. ISSN 2041-1723. PMC 7171104. PMID 32313096.
- ^ "GTDB release 09-RS220". Genome Taxonomy Database. Retrieved 10 May 2024.
- ^ "bac120_r220.sp_labels". Genome Taxonomy Database. Retrieved 10 May 2024.
- ^ "Taxon History". Genome Taxonomy Database. Retrieved 10 May 2024.
- ^ "The LTP". Retrieved 10 December 2024.
- ^ "LTP_all tree in newick format". Retrieved 10 December 2024.
- ^ "LTP_10_2024 Release Notes" (PDF). Retrieved 10 December 2024.













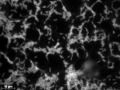



![Geobacter sulfurreducens and its bacterial nanowires[27]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d3/Proposal_of_catalyzing_bio-voltage_memristors.webp/120px-Proposal_of_catalyzing_bio-voltage_memristors.webp.png)
